AFP
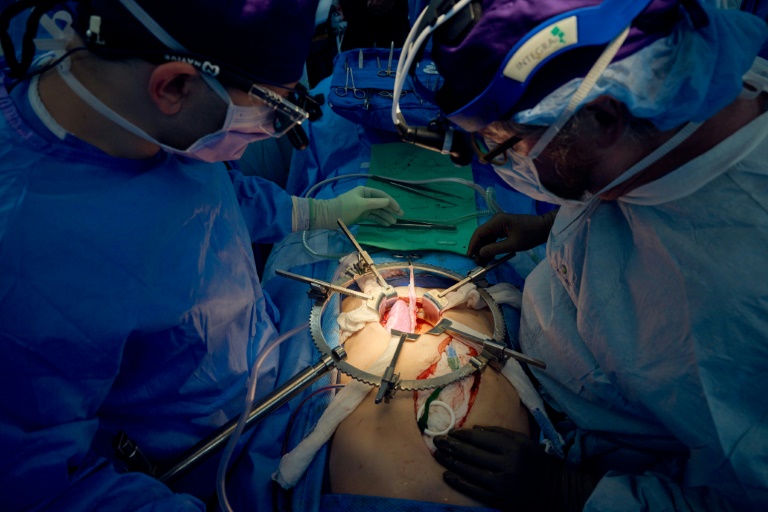
US surgeons who transplanted a genetically-modified pig kidney into a brain dead patient said Wednesday it was still working well after a record 32 days — a significant step in the quest to close the organ donation gap.
The latest experimental procedure is part of a growing field of research aimed at advancing cross-species transplants, using bodies that have been donated for science.
There are more than 103,000 people waiting for organs in the United States, 88,000 of whom need kidneys.
“We have a genetically-edited pig kidney surviving for over a month in a human,” Robert Montgomery, director of the NYU Langone Transplant Institute, told reporters. “I think there’s a very compelling story that exists at this point that I think should give further assurances about starting some initial studies… in living humans.”
Montgomery carried out the first genetically modified pig kidney transplant to a human in September 2021, followed by a similar procedure in November 2021. There have since been a handful of other cases, with all the experiments running for two or three days.
While previous transplants have involved up to 10 genetic modifications, the latest saw just one: in the gene involved in the so-called “hyperacute rejection,” which would otherwise occur within minutes of an animal organ being connected to a human circulatory system.
By “knocking out” the gene responsible for a biomolecule called alpha-gal — a prime target for roving human antibodies — the NYU Langone team were able to stop immediate rejection.
“We’ve now gathered more evidence to show that, at least in kidneys, just eliminating the gene that triggers a hyperacute rejection may be enough along with clinically approved immunosuppressive drugs to successfully manage the transplant in a human for optimal performance — potentially in the long-term,” said Montgomery.
They also embedded the pig’s thymus gland — which lies around the neck and is responsible for educating the immune system — in the kidney’s outer layer.
Adam Griesemer, of the NYU Grossman School of Medicine, added this allowed immune cells in the host’s body to learn to recognize the pig’s cells as its own, preventing a more delayed rejection.
Both of the patient’s own kidneys were removed, then one pig kidney was transplanted, and started immediately producing urine.
Monitoring showed that levels of creatinine, a waste product, were at optimal levels, and there was no evidence of rejection.
Crucially, no evidence of porcine cytomegalovirus — which may trigger organ failure — have been detected, and the team plan to continue monitoring for another month.
The research was made possible by the family of the 57-year-old male patient, Maurice “Mo” Miller, who was found unresponsive in his bathroom in July. Doctors determined he had an aggressive form of brain cancer, and would not wake up.
“Though my brother cannot be here, I can say with confidence he would be proud of the fact in the tragedy of his death, his legacy will be helping many people live,” his sister Mary Miller-Duffy told reporters.
In January 2022, surgeons at the University of Maryland Medical School carried out the world’s first pig-to-human transplant on a living patient. He died two months after the milestone, with the presence of porcine cytomegalovirus in the organ later blamed.
The donor pig came from a herd from Virginia-based biotech company Revivicor. The herd was approved by the Food and Drug Administration as a source of meat for people with hypersensitivity to the alpha-gal molecule, an allergy caused by tick bites.
These pigs are bred, not cloned, meaning the process can be more easily scaled.
Early xenotransplantation research focused on harvesting organs from primates — for example, a baboon heart was transplanted into a newborn known as “Baby Fae” in 1984, but she survived only 20 days.
Current efforts focus on pigs, which are thought to be ideal donors because of their organ size, their rapid growth and large litters, and the fact they are already raised as a food source.