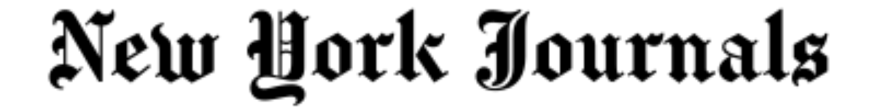A three-year-old girl has become the youngest patient in the UK to receive a groundbreaking gene therapy for a rare, life-threatening inherited condition.
Gunreet Kaur, from Hayes in west London, underwent the pioneering treatment for aromatic l-amino acid decarboxylase (AADC) deficiency, a disorder that severely impacts children’s physical, mental, and behavioural development.
Children afflicted with AADC deficiency typically struggle with fundamental bodily controls, including head movement, blood pressure regulation, and heart rate.
Gunreet was diagnosed with the condition when she was just nine months old, presenting a significant challenge for her family.
However, since receiving the new gene therapy, known as Upstaza, in February 2024, Gunreet has shown remarkable progress.
Her mother expressed astonishment at the advancements, which include new movements and vocalisation – milestones she once believed would be unattainable.
It is anticipated that Gunreet will continue to make further strides as she grows.
The innovative treatment was administered at the world-renowned Great Ormond Street children’s hospital (GOSH) in London. GOSH currently stands as the sole hospital in the UK offering this specific gene therapy to paediatric patients.
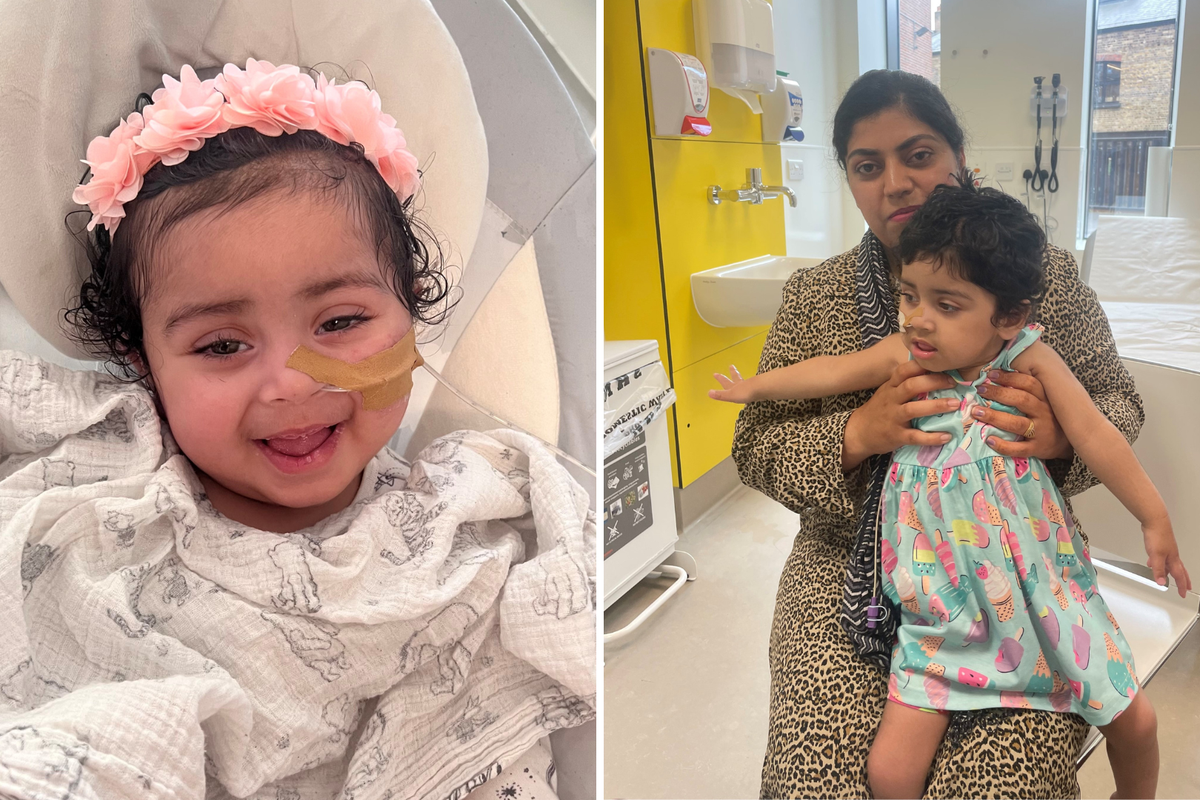
Sandeep Kaur said Gunreet has made “great progress” since her treatment.
“When Gunreet was about seven months old I noticed she wasn’t reaching her milestones at the same age that her older brother did,” she said.
“She couldn’t hold her own head up or reach out for items. She cried a lot and always wanted to be held.
“Since having the gene therapy, Gunreet has made great progress.
“She cries less, smiles more, and can reach for objects.
“She can hold her head up and is trying to sit up, she’s recently learned how to roll from her stomach to her back which is fantastic to see.
“It means a lot to me that Gunreet was able to have this gene therapy – her general health has improved, she has more co-ordination, she can bring her palms together and is able to move her hand to her mouth.”
AADC deficiency is caused by a mutation in the gene that produces the AADC enzyme, this enzyme is needed to produce a neurotransmitter called dopamine which is important in controlling movement.
People with AADC deficiency do not have a working version of the enzyme, which means that they have little or no dopamine in the brain.
This means that they can suffer developmental delays, weak muscle tone and inability to control the movement of the limbs. It can also lead to painful episodes for affected children.
The condition is rare and often deadly, with many children with AADC deficiency not reaching adulthood.
The medicine, also known as eladocagene exuparvovec, consists of a virus that contains a working version of the AADC gene.
The treatment is delivered by millimetre precision to an exact location in the brain of the patient by a team of medics assisted by a robotic surgery tool.
When given to the patient, it is expected that the virus will carry the AADC gene into nerve cells, enabling them to produce the missing enzyme.
This is expected to enable the cells to produce the dopamine they need to work properly, which will improve symptoms of the condition.
It is the first NHS England commissioned gene therapy in the UK infused directly into the brain.
Professor Manju Kurian, consultant paediatric neurologist at GOSH, said “AADC deficiency is a rare condition but often a cruel one that has such a profound impact on children and their carers and families.
“We know children with the condition have painful episodes that can last for hours and, as their condition progresses, their life becomes more and more difficult.
“It’s incredible to me that I can now prescribe novel gene therapies just as I would prescribe paracetamol and antibiotics.
“While the treatment is now available under the NHS at GOSH, we can only do this by working collaboratively across teams inside and outside the hospital, from physios and surgeons to dietitians and speech therapists, alongside partnerships with companies who supply these therapies.
“It’s great to see how this treatment has been able to help babies and children across the country like Gunreet.
“The natural history of the condition is that most patients cannot fully hold their head or make any developmental progress after that milestone.
“Gunreet’s progress over the last year has been really impressive in that context, as well as the virtual disappearance of the eye crises.
“We’re hopeful that one day she will be able to talk or walk, as seen in some of the young patients treated in the clinical trial.”
Professor James Palmer, NHS medical director for specialised commissioning, said: “This is wonderful news for Gunreet and her family, and a powerful example of how these commitments are translating into real improvements in people’s lives.
“By bringing cutting-edge medicines like eladocagene exuparvovec into the health service and setting up our expert clinical teams to successfully deliver them, the NHS is making clear its commitment to improving care through innovation.”