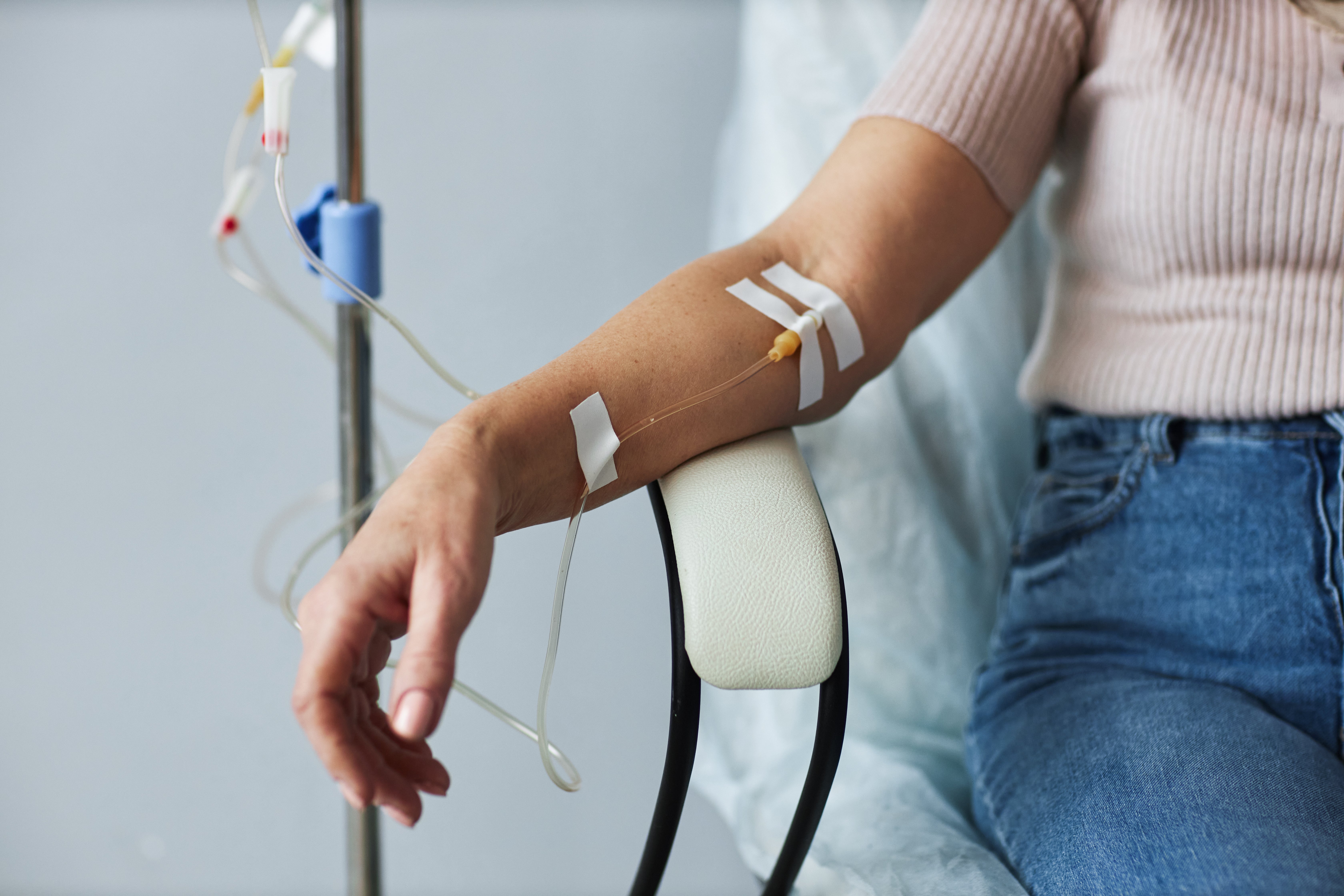
A consumer watchdog has issued a stark warning over the potentially “devastating” health consequences of intravenous (IV) drips marketed for skin lightening, as an investigation reveals hundreds of UK beauty establishments are offering the unregulated treatment.
These procedures, often referred to as skin whitening or brightening drips, contain the antioxidant glutathione, which is known for its skin-lightening properties.
However, glutathione used in this manner is not a licensed medicine in the UK, prompting experts to caution that it poses “significant safety risks” when administered by practitioners without proper medical oversight, such as beauticians.
The warning comes after a Channel 4 News investigation uncovered more than 300 beauty salons and clinics across the country offering these IV drips, with the vast majority operating without qualified doctors or nurses on staff.
In response to these findings, the Chartered Trading Standards Institute (CTSI) has urgently called for immediate regulation of these potentially dangerous procedures.
Kerry Nicol, external affairs manager at the CTSI, said: “I am truly shocked that these procedures are being carried out in the UK.
“These are not harmless beauty treatments – they are medical-style procedures being administered with no clinical oversight, no regulation, and no accountability.
“The consequences can be devastating. These procedures may be common overseas, but in the UK, we uphold the highest of safety standards and these products and procedures have no place on UK shores.
“We are calling for the urgent regulation of a sector that has the ability to cause great harm to consumers.”
Richard Knight, CTSI lead officer for cosmetics and beauty, added: “Whilst we in Trading Standards join with Environmental Health colleagues in seeking a concerted effort across government to deal with these treatments, the situation in the meantime is that consumers should only consider having any needle-based aesthetic treatments carried out by a qualified medical professional.”
Channel 4 found before-and-after videos on social media platforms of patients who had the treatment with visibly lighter skin.
It claims the price of these drips ranges from £75 to £100.
Campaign group Safety in Beauty warn that the treatments can cause strain on the liver and kidneys, allergic reactions, and infections from improper hygiene and injection techniques.
Andrew Rankin, a trustee at the Joint Council for Cosmetic Practitioners (JCCP), said: “These findings support our longstanding concerns.
“Glutathione used in this way is not a licensed medicine and presents significant safety risks when administered by unregulated practitioners.
“We urge the government to advance plans to license these procedures.”
Consultant dermatologist Dr Ophelia Dadzie told Channel 4 News is it “bewildering” that there is no regulatory oversight.
She claims to have raised concerns to the Medicines and Healthcare products Regulatory Agency (MHRA) as early as 2016.
“Until that changes, IV glutathione should not be offered at all,” Dr Dadzie added.
Lynda Scammell, head of borderlines at the MHRA, said: “Claims to lighten skin, around overall wellbeing or on boosting the immune system are not regarded as medicinal claims.
“We consider whether a product is a medicine or medical device on a strictly case-by-case basis taking account of legislation, guidance and appropriate case law.
“If a product is determined to be a medicinal product and is being offered for sale or supply without the appropriate authorisations, we will take appropriate action.”
A Department of Health and Social Care spokesperson urged anyone considering cosmetic procedures to think about the impact on health and ensure they use a reputable, insured and qualified practitioner.
“Work is ongoing to explore options around regulation of the non-surgical cosmetics sector and we will provide an update at the earliest opportunity,” they added.