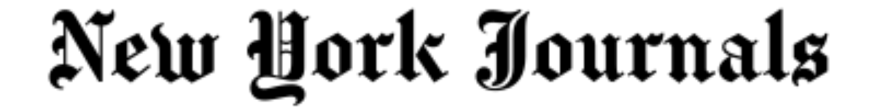A groundbreaking new drug that slows down the development of type 1 diabetes has been licensed for use in the UK.
Teplizumab can allow patients to live “normal lives” without the need for insulin injections.
Experts hailed the decision by the Medicines and Healthcare Regulatory Agency (MHRA) as a “breakthrough moment” that represents a “turning point” in how the condition is treated.
About 400,000 people in the UK have type 1 diabetes, a lifelong condition which causes the immune system to attack insulin-producing cells in the pancreas.
Insulin helps the body use sugar for energy, and without this hormone, blood sugar levels can become dangerously high.
Type 1 diabetes needs constant management to keep blood sugar within range, with patients required to take insulin through injections or pumps.
Teplizumab trains the immune system to stop attacking pancreatic cells.
It is taken by an IV drip for a minimum of 30 minutes over 14 consecutive days.
The drug, which is already approved in the US, has been authorised for use by the MHRA to delay the onset of stage three type 1 diabetes in adults and children aged eight or over by an average of three years.
Ahmed Moussa, general manager of general medicines UK and Ireland at Sanofi, which makes teplizumab, said: “One hundred years ago the discovery of insulin revolutionised diabetes care. Today’s news marks a big step forward.”
The UK is the first country in Europe to be granted a licence.
Type 1 diabetes develops gradually in three stages over months or years. Stage three is usually when people start to experience blood sugar problems and are diagnosed with the condition.
According to the MHRA, teplizumab is used in people with stage two type 1 diabetes, which is an earlier stage of the disease during which patients are at a high risk of progressing to stage three.
Parth Narendran, a professor of diabetes medicine at the University of Birmingham and The Queen Elizabeth Hospital Birmingham, said: “Teplizumab essentially trains the immune system to stop attacking the beta cells in the pancreas, allowing the pancreas to produce insulin without interference.
“This can allow eligible patients to live normal lives, delaying the need for insulin injections and the full weight of the disease’s daily management by up to three years. It allows people to prepare for disease progression rather than facing an abrupt emergency presentation.”
Following the decision by the MHRA, the cost-effectiveness of teplizumab will be assessed by NHS spending watchdog the National Institute for Health and Care Excellence (Nice) to determine if it can be rolled out on the health service.
Karen Addington, chief executive of the charity Breakthrough T1D, said: “I am personally delighted and welcome the MHRA’s approval of teplizumab.
“After years of research, clinical trials and drug development, we have an incredible breakthrough.”
Reacting to the announcement, Dr Elizabeth Robertson, director of research and clinical at Diabetes UK, said: “Today’s landmark licensing of teplizumab in the UK marks a turning point in the treatment of type 1 diabetes.
“For the first time, we have a medicine that targets the root cause of the condition, offering three precious extra years free from the relentless demands of managing type 1 diabetes.”
Dr Robertson added that the “next steps are critical”.
“To ensure teplizumab reaches everyone who could benefit, we need it to be made available on the NHS, and the rollout of a screening programme to identify those with early-stage type 1 diabetes,” she said.